Animal Research Facility
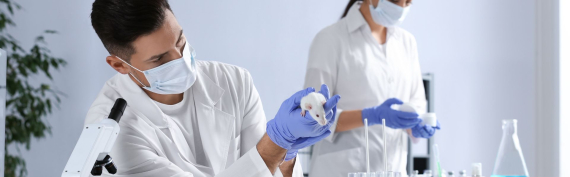
The Animal Research Facility (ARF) at PRC-ZU is the pioneering preclinical research center in Jordan, providing a wide range of high-quality services that support drug development and biomedical innovation. As the first facility of its kind in the country, the ARF offers comprehensive preclinical capabilities, including general toxicology, safety pharmacology, pharmacokinetics, efficacy testing, and customized studies. With a strong commitment to ethical practices, the ARF operates under the supervision of an established ethics committee, ensuring full compliance with international animal welfare guidelines.
We provide a comprehensive suite of preclinical research services, empowering you to confidently advance your novel compounds and therapies towards clinical trials. Our integrated Biological Research Unit, featuring a state-of-the-art animal facility and a sophisticated tissue culture laboratory, forms the cornerstone of our capabilities, allowing for seamless and efficient execution of a wide range of studies.
Integrated In Vivo Expertise: Bridging the Gap to Clinical Relevance
Our advanced animal facility is designed to support a diverse array of in vivo investigations. We are committed to the highest standards of animal welfare and ethical conduct, ensuring the integrity and reliability of your study outcomes. Our experienced team offers comprehensive support for:
Our preclinical services
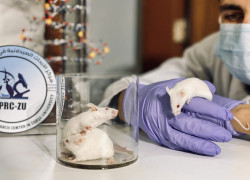
- In Vivo Toxicity Studies: We meticulously evaluate the safety profile of your compounds, determining maximum tolerated doses and identifying potential adverse effects across various animal models. Our rigorous protocols and detailed analysis provide critical insights for subsequent clinical development.
- Wound Healing Studies: Utilizing relevant animal models, we assess the efficacy of novel therapeutics in promoting tissue repair and regeneration. Our studies incorporate comprehensive endpoint analysis, including macroscopic and microscopic evaluation, to provide a thorough understanding of the healing process.
- Drug-Drug Interaction Studies: Understanding how investigational drugs interact with other medications is crucial for patient safety. We conduct carefully designed in vivo studies to elucidate potential pharmacokinetic and pharmacodynamic interactions, informing optimal dosing regimens and minimizing risks.
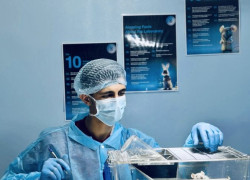
- Pharmacokinetic (PK) Studies: We provide detailed characterization of your compound's absorption, distribution, metabolism, and excretion (ADME) properties in vivo. Our expertise in various routes of administration and advanced bioanalytical techniques ensures accurate and timely PK data to guide dose selection and formulation strategies. In Vitro Precision: Unlocking Cellular Insights Our cutting-edge tissue culture laboratory complements our in vivo capabilities, offering a controlled environment for cellular and molecular investigations. We specialize in:
- Cytotoxicity Assays: We employ a range of established and customized assays to evaluate the potential of your compounds to induce cell death or inhibit cell growth. These studies provide valuable early-stage data for lead optimization and compound selection. Your Partner in Preclinical Success At Pharmaceutical Research Center- Zarqa University, we understand the critical importance of reliable and timely preclinical data. Our experienced scientific team, coupled with our state-of-the-art facilities, ensures the highest quality research and dependable results. We are committed to working collaboratively with you, providing tailored solutions to meet your specific research objectives and timelines. From study design and execution to comprehensive data analysis and reporting, we are your trusted partner in navigating the complexities of preclinical development.