PRC ZU (13)
Welcome to the Pharmaceutical Research Center at Zarqa University (PRC-ZU)
Written by Super UserWelcome to the Pharmaceutical Research Center at Zarqa University (PRC-ZU) – your hub for excellence in pharmaceutical research.
Founded in 2020 and recognized by the Ministry of Higher Education and Scientific Research, we aim to elevate the quality of scientific research, connect academia with industry, and meet the growing needs of pharmaceutical manufacturing companies in drug discovery and testing.
In 2023, we made history by becoming the first center in Jordan to receive approval from the Jordan Food and Drug Administration (JFDA) to conduct pre-clinical studies.
With our state-of-the-art facilities – including an Animal Research Facility, Analytical Laboratories, and a Cell Culture Laboratory – we provide a wide array of services to researchers and the industrial sector.
At PRC-ZU, we contribute to the advancement of pharmaceutical sciences and bridge the gap between academic knowledge and industry requirements. Whether you're a researcher seeking advanced facilities, a student aiming to enhance your skills, or a pharmaceutical company in pursuit of innovation, PRC-ZU is here to assist.
Explore our website to learn more about our facilities, services, research, and more.
Email:
This email address is being protected from spambots. You need JavaScript enabled to view it.
Phone:
+96253821100
Ext:
2130-2131
Location:
Zarqa University / Zarqa-Jordan
Overview
At Zarqa University's Pharmaceutical Research Centre (PRC-ZU), our Quality Assurance Department forms a crucial aspect of our research and development process. With an unwavering commitment to upholding the utmost standards of research, safety, and efficacy, our goal is to ensure that all activities align with international standards and regulations, as well as university guidelines. A team of committed professionals partners with researchers, lab staff, and other stakeholders, guaranteeing the validity, reliability, and honesty of research outcomes.
Our Quality Management Framework
PRC-ZU's QA Department adopts a robust Quality Management System (QMS) to persistently supervise and enhance the Centre's research procedures, thereby ascertaining superior quality research. This strategy hinges on globally acknowledged quality standards, inclusive of Good Laboratory Practices (GLP), which we stringently apply. Alongside GLP, we also adhere to Good Documentation Practices (GDP) and Good Manufacturing Practices (GMP). Our meticulous and exhaustive documentation systems and regular audits confirm that all activities are accurately documented, with data easily accessible for review and scrutiny. Our QMS also incorporates training initiatives for researchers and staff to stay abreast of the latest industry protocols and innovations.
JFDA Approval and Adherence to GLP Standards
We are proud to announce that our center has achieved a significant milestone in its journey toward excellence. The Pharmaceutical Research Centre at Zarqa University has earned the approval of the FDA to conduct pre-clinical studies. This approval came after an exhaustive inspection of our center, conducted according to the stringent standards of Good Laboratory Practices (GLP). This not only validates our unwavering commitment to adhering to globally recognized quality standards but also reinforces our dedication to advancing impactful and groundbreaking research in pharmaceuticals. The FDA's approval further strengthens our resolve to continue delivering high-quality, reliable, and innovative pre-clinical studies.
A Cooperative Approach to Quality Assurance
At the core of our QA Department's methodology lies collaboration. By fostering an environment of open dialogue and teamwork, we are able to maintain premier levels of quality and safety. Our QA team collaborates extensively with PRC-ZU's various research divisions and external associates, ensuring our research methodologies remain transparent and compliant. Through consistent meetings, workshops, and mutual assessments, we continually appraise and refine our processes to contribute to the creation of safe and efficient pharmaceutical products capable of enhancing patient lives worldwide.
Striving for ISO 17025 Accreditation
In our pursuit to offer clients superior research services, PRC-ZU is working towards achieving ISO 17025 accreditation—an internationally recognized standard for testing and calibration laboratories. By obtaining ISO 17025 accreditation, we can offer our clients an additional layer of confidence that our laboratories meet the highest standards of technical competence, impartiality, and consistency. We are confident that this accreditation will further strengthen our standing in the field of pharmaceutical research, reinforcing our commitment to excellence.
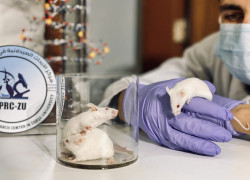
Pioneering Animal Research Facility
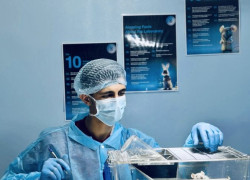
The ARF is the pioneering preclinical animal research facility in Jordan. We specialize in conducting preclinical services involving animal experiments, strictly adhering to animal protection and welfare standards. Our facility is open to both internal and external researchers from Zarqa University, as well as companies from the pharmaceutical, cosmetics, and chemical industries.
Ethical Practices and Comprehensive Procedures
With swift protocol review processes in place, the ARF has established a comprehensive ethics committee for the care and utilization of animals in scientific research. This committee convenes regularly to supervise the care and usage of research animals. The ARF-PRC-ZU's extensive processes and procedures include animal acclimation, dosing, observations, data recording, pathology, ensuring data integrity, and report writing.
Seamless Transition to Clinical Studies
Our preclinical and bioanalytical services collaborate closely at the ARF to provide customers with precise and trustworthy results, enabling a seamless and expedient transition from preclinical to clinical studies.
Proficient Team
The ARF team is constituted of skilled and proficient bio-scientists and pharmacy professionals. They employ their extensive knowledge and experience to contribute to the development of next-generation medications and therapeutics.
Customer-Focused Approach
Our team at ARF is cognizant of the risks and time constraints associated with preclinical services. We aim to minimize any associated distress by delivering quality services that exceed expectations. Customer satisfaction and the integrated quality of animal studies serve as our primary driving force, while we strive to keep our pricing affordable.
Preclinical Services
We offer a wide range of preclinical services, including:
General Toxicology
Safety Pharmacology
Pharmacokinetics
Efficacy Testing
And much more...